Every single person carries within them a biological ID system, a silent code that dictates not only their ancestry but, more immediately, the very mechanics of their survival in a critical medical scenario. This ID, the blood type, isn’t just a random letter; it’s a detailed, intricate fingerprint of protein molecules nestled on the surface of your red blood cells. Ignoring this biological reality in a medical setting is unthinkable—it’s the difference between a life-saving intervention and a sudden, catastrophic immune reaction. While we commonly talk about the simple letters—A, B, AB, and O—the actual story is far more layered, involving two major classification systems, the ABO system and the Rh factor, that interact in ways that create eight common, yet uniquely specific, types. To truly grasp the complexity of compatibility during a blood transfusion, you have to move beyond the surface-level labels and understand the core mechanism: the chemical warfare between antigens and antibodies. This war, fought entirely within the bloodstream, is the reason a simple mistake in blood matching can lead to an immediate and severe immune response, where the transfused blood cells are rapidly destroyed by the recipient’s own defense system. It’s a beautifully simple, yet brutally unforgiving, set of biochemical rules that governs who can safely accept life-saving donations.
The chemical warfare between antigens and antibodies.
To start peeling back the layers of this system, we need to focus on what makes your blood yours. It comes down to antigens. These are specific protein markers that reside on the surface of your red blood cells, acting like little flags that tell your immune system, “This cell belongs here.” The ABO system is governed by the presence or absence of two main flags: the A antigen and the B antigen. For example, if you have Type A blood, your red cells proudly display the A antigen. Conversely, if you have Type B blood, you display the B antigen. A person with Type AB blood has both A and B antigens, making their cells look acceptable to both A and B environments. The individual with Type O blood has neither the A nor the B antigen, presenting a kind of blank slate. The corresponding defense mechanism is the antibody, found floating in your blood plasma. These antibodies are the immune system’s sharp-shooters; they are programmed to attack any antigen that they do not recognize as belonging to the body. This sets up the critical conflict: an A individual carries anti-B antibodies, ready to destroy any B-type cells introduced, and vice versa. It is this fundamental, pre-programmed conflict that dictates the immediate and absolute rule of compatibility.
An A individual carries anti-B antibodies, ready to destroy any B-type cells introduced.
The entire safety protocol of a blood transfusion is built on avoiding the tragic scenario known as an acute haemolytic transfusion reaction. This occurs when incompatible blood is introduced, causing the recipient’s pre-existing antibodies to instantly bind to the donor’s foreign red blood cells. When this binding happens, the immune response is swift and brutal: the donor cells are rapidly clumped together and destroyed (a process called agglutination and hemolysis). The consequences are devastating, leading to kidney failure, shock, and potentially death. For this reason, compatibility testing isn’t just a suggestion; it is the most critical checkpoint in emergency medicine. Understanding the direct threat posed by incompatible antibodies helps explain why certain types are restricted. If you have Type A blood, receiving Type B blood is fundamentally lethal because your anti-B antibodies will immediately recognize the B antigens as foreign invaders and launch a full-scale assault. The body is simply doing its job of defense, but in this context, that defense mechanism is fatal to the treatment. This is why the universal donor and universal recipient labels are so important to memorize, as they represent the exceptions to this high-stakes immune scrutiny.
The entire safety protocol of a blood transfusion is built on avoiding the tragic scenario known as an acute haemolytic transfusion reaction.
Beyond the initial A, B, AB, and O classification, there exists another critical factor that dramatically increases the complexity of compatibility: the Rh factor. The Rh system is named after the Rhesus monkey, where this protein was first identified, but it is a ubiquitous part of human blood chemistry. Essentially, the Rh factor is another type of antigen—the D antigen—that either is, or isn’t, present on your red blood cells. If you have the Rh antigen, you are considered Rh positive (Rh+), such as A+ or O+. If you do not have this antigen, you are Rh negative (Rh−), such as A− or O−. This single protein doubles the number of common blood types from four to eight, creating a necessary additional layer of scrutiny during any transfusion. The Rh factor’s incompatibility differs from the ABO system in one important aspect: an Rh− individual doesn’t usually possess pre-formed anti-Rh antibodies unless they have been previously exposed to Rh+ blood, such as through a prior incompatible transfusion or during pregnancy. This delayed reaction potential makes the Rh system a subtle, yet equally serious, concern in medical practice.
The Rh factor’s incompatibility differs from the ABO system in one important aspect.
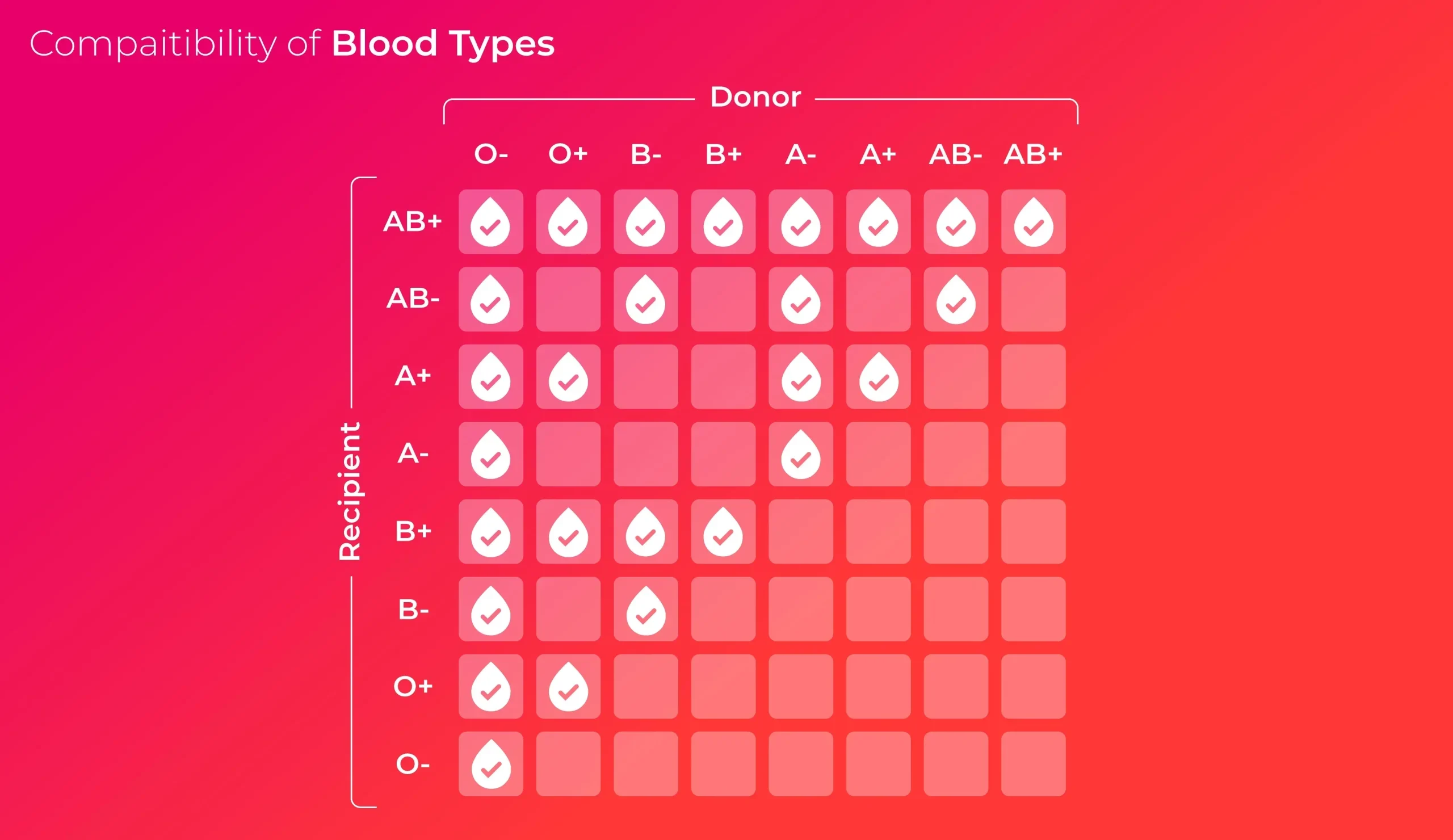
The practical implication of the Rh factor is perhaps most vividly seen in the designation of the universal donor. While Type O blood lacks the A and B antigens, O− blood specifically lacks all three major antigens—A, B, and Rh. This makes it an incredibly precious commodity in emergency situations. Because O− blood carries none of the flags (antigens) that an immune system is pre-programmed to fight against, it can, theoretically, be given safely to anyone, regardless of their own type. It is the blank slate of the blood world, an immediate lifeline when a patient’s blood type is unknown and time is of the essence. However, the designation “universal donor” only applies to the red blood cells themselves. For plasma, the compatibility rules are effectively flipped, adding a further layer of complexity that is often overlooked in general discussions. Understanding this critical distinction between red blood cell and plasma compatibility is vital for the holistic management of blood product inventories in a hospital setting.
O- blood specifically lacks all three major antigens—A, B, and Rh.
The opposite end of the compatibility spectrum is held by AB+ blood, which is correctly labeled the universal recipient for red blood cells. A person with AB+ blood possesses the A antigen, the B antigen, and the Rh antigen. Because their red cells display all three of these major flags, their immune system is wired to recognize all of them as “self.” Crucially, this means they do not develop antibodies against A, B, or Rh proteins. Consequently, they can receive red blood cells from any other ABO or Rh type—A+, B−, O+, or anything in between—without suffering an immediate immune reaction. While this is an enormous benefit to the patient with AB+ blood, making the search for donor blood much easier for them, it also means that their blood cannot be widely donated, as it carries antigens that many other types would immediately reject. The rarity of this blood type, despite its recipient flexibility, makes its specific collection and allocation an important logistical task for blood banks.
A person with AB+ blood possesses the A antigen, the B antigen, and the Rh antigen.
The inheritance of blood type, determined by the alleles passed down from both parents, often creates family discussions and occasional confusion when a child’s blood type doesn’t immediately match either parent’s, demonstrating the subtle complexity of human genetics. The ABO gene has three major alleles: A, B, and O. The A and B alleles are co-dominant, meaning if you inherit one of each, you express both antigens (Type AB). The O allele is recessive, meaning it only expresses itself (Type O) if two O alleles are inherited. The existence of the recessive O allele explains how two Type A parents (each having an A and an O allele, AO) can suddenly produce a Type O child (by passing on their recessive O alleles, resulting in OO). This is a perfect illustration of how genetics can hide and reveal traits across generations, and it’s a necessary factor to consider when navigating certain medical and paternity questions, though blood type is only one piece of a much larger genetic puzzle.
The existence of the recessive O allele explains how two Type A parents can suddenly produce a Type O child.
The Rh factor introduces a unique and serious challenge during pregnancy, especially when an Rh− mother is carrying an Rh+ fetus, a common scenario when the father is Rh+. The initial pregnancy is usually not a problem, but during delivery, a small amount of the baby’s Rh+ blood can mix with the mother’s circulation. Her immune system, recognizing the Rh antigen as foreign, will begin to produce anti-Rh antibodies. The real danger lies in subsequent pregnancies involving Rh+ babies. These antibodies, once formed, can cross the placenta and attack the fetus’s red blood cells, leading to a condition called Hemolytic Disease of the Newborn (HDN), which can be life-threatening. Thankfully, modern medicine provides a solution in the form of RhoGAM, an injection given to Rh− mothers that prevents the formation of these dangerous antibodies, effectively neutralizing the immune system’s potential attack and safeguarding future pregnancies. This intervention is a testament to the life-saving application of understanding complex immunologic differences.
The real danger lies in subsequent pregnancies involving Rh+ babies.
While the most obvious application of blood type knowledge is in transfusion, the antigens and related genetic markers are also being studied for their potential, albeit highly debated, links to other areas of health. Researchers have been looking at potential associations between specific blood types and the risk or resistance to certain diseases, including vulnerabilities to specific types of infectious agents or even cardiovascular events. These studies are often complex and the findings are rarely definitive, but they underscore the pervasive influence of these small cellular markers. For instance, some research suggests a potential correlation between Type O blood and a slightly lower risk of certain cardiovascular conditions, though this is not a firm rule and should not replace well-established lifestyle health advice. This growing area of inquiry takes the simple ABO label and elevates it from a mere transfusion necessity to a potentially informative piece of a person’s overall biological profile, though the science remains cautious and evolving.
This growing area of inquiry takes the simple ABO label and elevates it from a mere transfusion necessity to a potentially informative piece of a person’s overall biological profile.
Ultimately, understanding blood types is an exercise in appreciating the body’s highly specific and non-negotiable chemical boundaries. It’s not just a set of arbitrary rules but a reflection of evolutionary necessity—the immune system’s perfected ability to distinguish self from non-self. For the person donating blood, knowing their type—especially if it is O− or AB plasma—allows them to contribute uniquely to the medical supply chain. For the patient, it is the fundamental assurance of safety during a critical procedure. The careful cross-matching done in a lab is the unglamorous, high-stakes application of this knowledge, where every test tube represents a life that hangs in the balance of a perfectly respected biochemical boundary. This system, built on tiny antigens and powerful antibodies, remains one of the cornerstones of modern healthcare, demanding absolute precision and unwavering respect for its rules.
The careful cross-matching done in a lab is the unglamorous, high-stakes application of this knowledge.
The ABO and Rh systems, along with many other minor blood groups, are more than just textbook facts; they are the cellular architecture defining life-saving compatibility, demanding absolute precision to prevent an immune system revolt.
